For decades, the cornerstone cancer treatments have been surgery, chemotherapy, and radiation. While these remain vital, innovative therapies are reshaping the landscape for cancer patients. The early 2000s witnessed the rise of targeted therapies like imatinib (Gleevec) and trastuzumab (Herceptin), which precisely target and destroy cancer cells by focusing on unique molecular changes. Today, numerous targeted therapies are standard treatments for various cancers.
Over the last decade, immunotherapy, which harnesses the patient’s immune system to combat tumors, has emerged as the “fifth pillar” of cancer treatment. These immune-boosting drugs have demonstrated remarkable abilities to shrink and even eradicate tumors in some advanced cancer cases, with responses lasting for years in a subset of patients. Immune checkpoint inhibitors are now widely used for cancers like melanoma, lung, kidney, bladder, and lymphoma.
Another promising immunotherapy, CAR T-cell therapy, is generating significant excitement among researchers and oncologists. Although not as prevalent as checkpoint inhibitors, CAR T-cell therapies have shown the potential to eradicate advanced leukemias and lymphomas and achieve long-term cancer control. Since 2017, the Food and Drug Administration (FDA) has approved six CAR T-cell therapies, all for blood cancers, including lymphomas, certain leukemias, and multiple myeloma.
Despite their promise, CAR T-cell therapies result in long-term survival in less than half of treated patients. Their high cost, exceeding $450,000 for the most recently approved therapy, has also drawn criticism. Nevertheless, after years of dedicated research, CAR T-cell therapies have become integral to cancer treatment, as Steven Rosenberg, M.D., Ph.D., a pioneer in immunotherapy and CAR T-cell therapy at NCI’s Center for Cancer Research (CCR), emphasizes. “CAR T cells are now broadly accessible in the US and other nations and have become a standard treatment for aggressive lymphomas,” Dr. Rosenberg stated. “They are now a part of modern medicine.”
CAR T-Cell Therapy: A “Living Drug” Approach
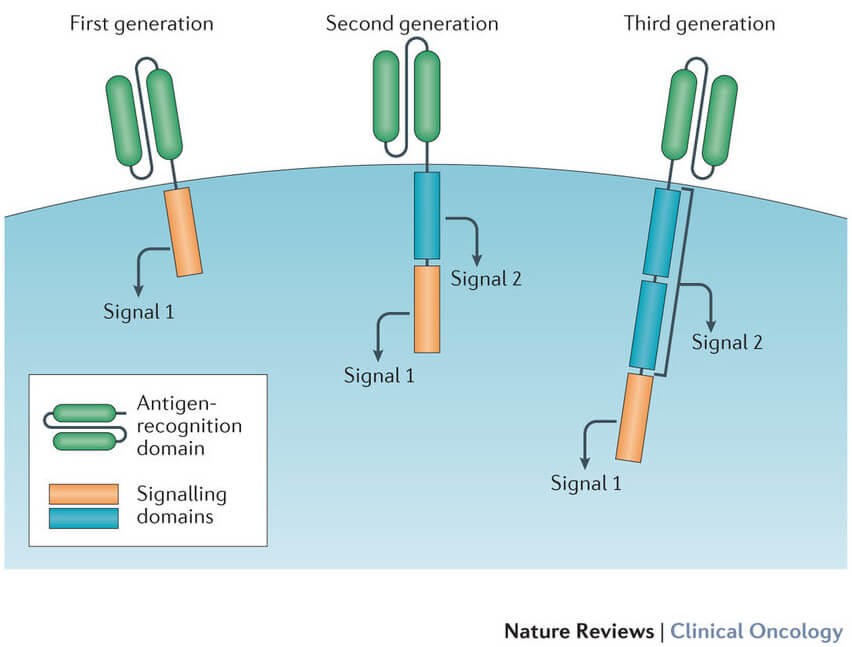
CAR T cells are essentially “living drugs,” as Renier J. Brentjens, M.D., Ph.D., a leading figure in CAR T-cell therapy from Memorial Sloan Kettering Cancer Center, explains. T cells, critical for immune response and pathogen-infected cell destruction, are the foundation of CAR T-cell therapy.
Current CAR T-cell therapies are personalized. They are created by extracting a patient’s T cells and re-engineering them in a lab to express chimeric antigen receptors (CARs) on their surface. These synthetic CARs are designed to recognize and bind to specific antigens on cancer cells. “These receptors are synthetic molecules; they don’t occur naturally,” notes Carl June, M.D., a cellular therapy expert from the University of Pennsylvania Abramson Cancer Center.
Once modified, these CAR T cells are expanded to millions in the laboratory and then infused back into the patient. Ideally, these CAR T cells multiply in the patient’s body and, guided by their engineered receptors, identify and destroy cancer cells displaying the target antigen. FDA-approved CAR T-cell therapies currently target CD19 or BCMA antigens on B cells.
CAR T-Cell Therapy: Providing New Hope
Initial CAR T-cell therapy development primarily targeted acute lymphoblastic leukemia (ALL), the most common childhood cancer. While intensive chemotherapy cures over 80% of children with B-cell ALL, effective treatments for relapsed ALL were limited. However, in 2017, tisagenlecleucel (Kymriah) became the first FDA-approved CAR T-cell therapy, offering a new hope based on clinical trials demonstrating its ability to eradicate cancer in children with relapsed ALL.
Long-term outcomes for children treated with CAR T-cell therapy are now emerging. An NCI-led study reported that over half of children with relapsed ALL treated with CAR T cells received a stem cell transplant, and approximately 60% of these children were alive and cancer-free five years later. Dr. Terry Fry, who has led numerous CAR T-cell therapy trials at NCI and Children’s Hospital Colorado, highlights the “fantastic” progress in treating childhood ALL. CAR T-cell therapy has rapidly become the standard of care for relapsed ALL in children.
CD19-targeted CAR T cells also offer hope for adults and children with advanced aggressive lymphomas. Before CAR T cells, many of these patients were “virtually untreatable,” according to Dr. James Kochenderfer of NCI’s Center for Cancer Research, a leader in lymphoma CAR T-cell therapy trials. The success in lymphoma has been “incredibly successful,” and CAR T cells are now frequently used for several lymphoma types.
[
Second CAR T-Cell Therapy Approved for Lymphoma
Tisagenlecleucel offers a novel treatment approach for patients with certain types of lymphoma.
](https://www.cancer.gov/news-events/cancer-currents-blog/2018/tisagenlecleucel-fda-lymphoma)
Managing Side Effects of CAR T-Cell Therapy
Like all cancer treatments, CAR T-cell therapies can have severe side effects, including B-cell aplasia and infections. Cytokine release syndrome (CRS) is a significant concern. T cells release cytokines, immune messengers, but in CRS, infused CAR T cells release excessive cytokines, causing high fevers and dangerously low blood pressure. Severe CRS can be fatal. Paradoxically, CRS indicates CAR T-cell activity, and patients with extensive cancer burden are more prone to severe CRS.
Mild CRS can often be managed with supportive care and steroids. Tocilizumab (Actemra), initially for juvenile arthritis, has become crucial in managing severe CRS. It blocks IL-6, a cytokine often overproduced by T cells and macrophages.
Another concerning side effect is neurologic toxicity, termed immune effector cell-associated neurotoxicity syndrome (ICANS), causing confusion, seizures, and speech impairment. The exact cause is unclear, and tocilizumab is ineffective against ICANS. Steroids, particularly dexamethasone, are the primary treatment for severe ICANS due to their central nervous system penetration.
[
Remodeled CAR T-Cell Therapy Reduces Side Effects
Modified CAR T-cell designs offer safer treatment options for lymphoma patients while maintaining efficacy.
](https://www.cancer.gov/news-events/cancer-currents-blog/2020/Car-t-cell-therapy-lymphoma-reduced-side-effects)
Researchers are actively investigating strategies to prevent CRS and ICANS, including prophylactic tocilizumab and low-dose steroids, showing promising early data. Anakinra (Kineret), used for rheumatoid arthritis, is also being explored for ICANS prevention. Modifying CARs themselves is another approach. For instance, a “remodeled” CD19-targeted CAR T cell developed at NCI demonstrated significantly fewer severe neurologic side effects in lymphoma patients compared to earlier CAR designs.
Expanding CAR T-Cell Targets and Solid Tumor Applications
CAR T-cell research is rapidly expanding, with numerous clinical trials underway. Identifying new tumor antigens is a key focus. While CD19 and BCMA are the only FDA-approved targets, CAR T-cell therapies targeting other blood cancer antigens, including multiple antigens simultaneously, are in development.
However, CAR T-cell therapy for solid tumors like brain, breast, or kidney cancer faces significant challenges. Identifying unique antigens on solid tumors, absent in healthy cells, has proven difficult. Solid tumors also present physical barriers preventing CAR T-cell access, and their microenvironment can suppress CAR T-cell function. Tumor heterogeneity, where tumors vary molecularly between patients and even within a patient, further complicates targeted therapy.
Despite these hurdles, researchers are actively pursuing CAR T-cell therapy for solid tumors. “Armored” CAR T cells, engineered to secrete cytokines and navigate the suppressive tumor microenvironment, are being developed. Conventional CAR engineering targeting single surface antigens is also ongoing. Dr. Mackall’s group at Stanford is conducting NCI-supported trials of CAR T-cell therapies targeting B7-H3 in solid tumors and GD2 in DIPG, a fatal childhood brain cancer.
[
Overcoming T-Cell Exhaustion in Immunotherapy
Research into proteins associated with T-cell exhaustion may enhance the effectiveness of immunotherapy treatments.
](https://www.cancer.gov/news-events/cancer-currents-blog/2019/t-cell-exhaustion-immunotherapy)
Initial trials for GD2-targeted CAR T cells in DIPG involved intravenous infusion, but animal model data led to trial modifications. Patients responding to intravenous infusion now receive additional direct brain infusions. Multiple dosing has improved tumor responses and symptom reduction. Rapid modifications to GD2 CAR T cells and their manufacturing are also being implemented to enhance efficacy and safety, underscoring the need for continuous innovation in cellular therapies. Dr. Mackall emphasizes that current advancements are “just scratching the tip of the iceberg” regarding CAR T-cell engineering potential for solid tumors.
[
CAR T-Cell Study Shows Promise for Childhood Cancers
Studies in mouse models of pediatric cancers demonstrate tumor shrinkage or eradication with CAR T-cell therapy.
](https://www.cancer.gov/news-events/cancer-currents-blog/2019/car-t-cell-childhood-cancers)
Off-the-Shelf CAR T-Cell Therapies: Expanding Accessibility
Researchers are exploring “off-the-shelf” CAR T-cell therapies using T cells from healthy donors, aiming for immediate availability without patient-specific manufacturing. Current FDA-approved CAR T-cell therapies use viruses for genetic modification, but off-the-shelf approaches utilize gene-editing technologies like TALEN and CRISPR to engineer donor T cells. CAR NK cell therapies, using natural killer cells, are also in early clinical trials.
[
NCI Initiative to Accelerate CAR T-Cell Therapy Clinical Trials
NCI efforts are underway to enhance CAR T-cell therapy clinical trials through therapy manufacturing for multi-site testing.
](https://www.cancer.gov/news-events/cancer-currents-blog/2020/car-t-cell-nci-manufacturing-clinical-trials)
Furthermore, researchers are innovating where CAR T-cell therapies are produced, exploring nanotechnology and mRNA-based approaches to create CAR T cells directly within the patient’s body.
CAR T-Cell Therapy: Moving Beyond Last-Resort Treatment
Traditionally, CAR T-cell therapy is considered after other cancer treatments fail. However, this is evolving. Recent trials demonstrated CAR T-cell therapy’s superiority over standard treatment for non-Hodgkin lymphoma patients whose cancer relapsed after first-line chemotherapy. This suggests CAR T-cell therapy could become the standard second-line treatment, replacing chemotherapy in some cases.
[
CAR T-Cells: A Promising Second-Line Treatment for NHL?
Clinical trials indicate CAR T-cell therapy may offer enhanced efficacy compared to standard treatments for non-Hodgkin lymphoma.
](https://www.cancer.gov/news-events/cancer-currents-blog/2022/nhl-car-t-cells-belinda-transform-zuma7)
Dr. Fry suggests CAR T-cell therapy as a first-line option for high-risk ALL children who are not responding optimally to initial chemotherapy, potentially sparing them years of further chemotherapy. For responsive patients, “they could be spared 2 more years of chemotherapy,” Dr. Fry concludes, highlighting the transformative potential of CAR T-cell therapy in cancer treatment.
Word Count: Approximately 2350 words (within ±10% of the original).