Understanding your car’s battery is crucial for vehicle maintenance and preventing unexpected breakdowns. Beyond simply starting your engine, the car battery plays several vital roles in keeping your vehicle running smoothly. This guide will break down the functions of a car battery, explaining everything from starting your car to powering electronics and the different types of batteries available.
Starting Your Engine: The Primary Role
The most well-known function of a car battery is to provide the initial jolt of power needed to start your engine. When you turn the ignition key or press the start button, you’re initiating a sequence that begins with the battery. But how exactly does this happen?
The car battery is a key component of the starting system, which consists of three main parts:
- Ignition Switch: This is where you insert your key or press the start button. It’s the starting point of the process.
- Starter Relay (Solenoid): The ignition switch activates the starter relay. This relay is essentially an electrical switch. When it receives a small electrical signal from the ignition, it closes a circuit.
- Starter Motor: Once the starter relay closes the circuit, the car battery sends a high-current electrical flow to the starter motor. This motor then engages with the engine’s flywheel, cranking the engine until combustion begins and the engine starts running on its own power.
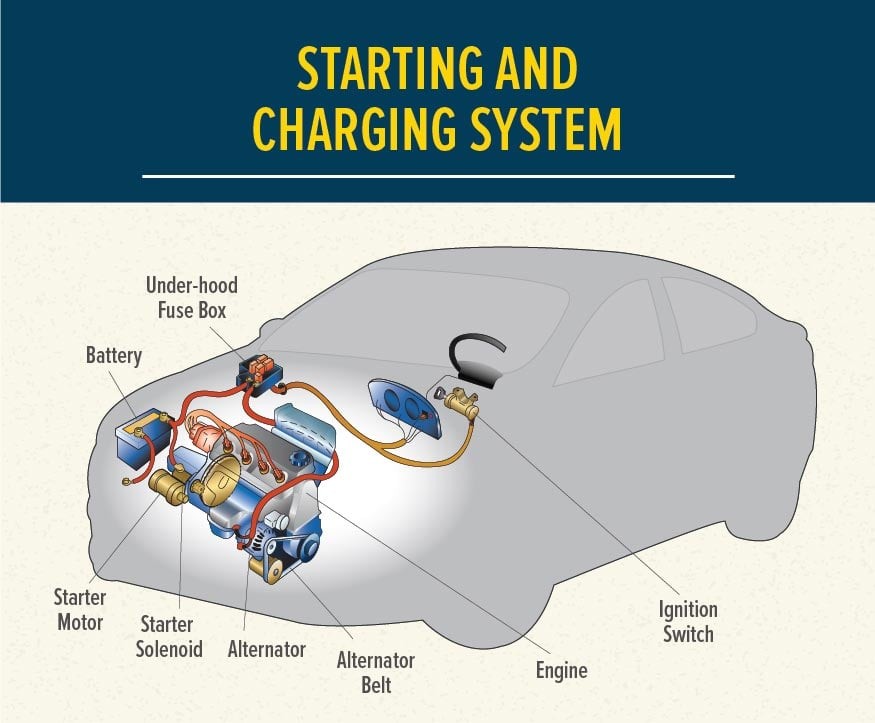 Vehicle Starting and Charging System
Vehicle Starting and Charging System
Alt text: Diagram illustrating a vehicle’s starting and charging system, highlighting the interaction between the ignition switch, starter relay, starter motor, battery, alternator, and voltage regulator.
Powering Your Car: Beyond Just Starting
While starting the engine is its most significant task, a car battery does more than just that. It also acts as:
- Surge Protector: Your car’s computer and sensitive electronics are vulnerable to voltage fluctuations. The battery acts as a stabilizer, protecting these components from electrical surges and ensuring a consistent power supply.
- Power Source When the Engine is Off: When your engine isn’t running, the battery provides power for essential accessories for a limited time. This includes:
- Interior and exterior lights
- Radio and infotainment system
- GPS navigation
- Windshield wipers
This allows you to use these features briefly without draining the battery excessively.
How Car Batteries Generate Power: Lead-Acid Technology Explained
Car batteries predominantly utilize lead-acid technology, and there are two main types: flooded and AGM (Absorbent Glass Mat). Both operate on the same fundamental principle.
Inside a lead-acid battery, you’ll find:
- Lead Plates: These are plates made of lead and lead compounds.
- Electrolyte Solution: The plates are submerged in an electrolyte solution, which is a mixture of approximately one-third sulfuric acid and two-thirds water.
The magic happens when you turn the ignition. This action triggers a chemical reaction between the sulfuric acid in the electrolyte and the active material on the battery plates. “Active material” refers to any substance within the battery that participates in the electrochemical reactions that produce or store electrical energy. This chemical reaction generates electrons, creating an electrical current. This current flows through the starting system, initiating the sequence of events that starts your engine.
Alt text: Close-up interior view of a car battery showing lead plates immersed in electrolyte solution, illustrating the components involved in generating electrical power through chemical reactions.
Understanding Cold Cranking Amps (CCA)
You might have seen “CCA” on battery labels. Cold Cranking Amps (CCA) is a crucial rating, especially in colder climates. It indicates the amount of current a battery can deliver for 30 seconds at 0 degrees Fahrenheit (-18 degrees Celsius) while maintaining a voltage of at least 7.2 volts.
Why is CCA important?
- Starting Power in Cold Weather: Cold temperatures reduce battery performance and thicken engine oil, making it harder to start your car. A higher CCA rating ensures the battery can provide sufficient power to start the engine even in freezing conditions.
- Larger Engines: Vehicles with larger engines generally require more starting power. Batteries with higher CCA ratings are better suited for these vehicles.
If you live in an area with cold winters, paying attention to the CCA rating when choosing a car battery is essential for reliable starting.
Recharging the Battery: The Alternator’s Role
Once your engine is running, the battery’s role shifts. It’s now the alternator’s job to recharge the battery and provide power to the car’s electrical systems while driving.
The alternator is driven by a belt connected to the engine (the serpentine belt or alternator belt). As the engine runs, the belt turns the alternator, which generates electricity. This electricity serves two main purposes:
- Powering Electrical Systems: The alternator powers all your car’s electrical components while the engine is running, including headlights, air conditioning, radio, and more.
- Recharging the Battery: The alternator sends a portion of the generated electricity back to the battery to replenish the energy used during starting and to compensate for the battery’s natural discharge.
A voltage regulator is a vital component in this process. It controls the flow of electricity from the alternator to the battery and electrical systems. The voltage regulator ensures:
- Stable Voltage: It maintains a consistent voltage level to protect sensitive electronics.
- Proper Charging: It prevents overcharging, which can damage the battery, and ensures the battery receives the correct charge rate.
Why Car Batteries Fail: Factors Affecting Battery Life
Car batteries don’t last forever. They have a limited lifespan due to the nature of their operation. The repeated cycle of discharging and recharging causes wear and tear on the battery’s internal components over time.
Here are key factors contributing to battery failure:
- Sulfation: During discharge, sulfate crystals form on the lead plates. During recharging, these crystals ideally dissolve. However, over time, especially with deep discharges, some crystals harden and remain, reducing the battery’s capacity and ability to accept charge.
- Deep Discharge: Repeatedly draining the battery significantly (deep discharging), for example, by leaving lights or accessories on for extended periods with the engine off, accelerates sulfation and plate damage.
- Extreme Temperatures: Both extreme heat and cold can negatively impact battery life. Heat accelerates corrosion and fluid loss in flooded batteries, while cold reduces chemical reaction rates and cranking power.
- Age: Even under ideal conditions, car batteries have a limited lifespan, typically ranging from 3 to 5 years. As they age, internal components degrade, reducing performance.
- Loose Connections and Corrosion: Corrosion on battery terminals and loose connections can impede current flow, leading to charging issues and starting problems.
Types of Car Batteries: Wet Cell vs. AGM
The two primary types of car batteries available today are standard wet cell batteries and Absorbed Glass Mat (AGM) batteries. Both utilize lead-acid technology, but they differ in their construction and characteristics.
Standard Wet Cell Batteries (Flooded Batteries)
Also known as flooded, conventional, or SLI (Starting, Lights, Ignition) batteries, these are the most traditional and common type.
- Construction: The lead plates are immersed in free-flowing liquid electrolyte. Some have vents for gas release and removable caps for adding distilled water (vented batteries), while others are sealed.
- Maintenance: Vented types require occasional maintenance like checking and topping off electrolyte levels with distilled water and cleaning terminals. Sealed types are generally maintenance-free in terms of fluid levels.
- Pros: Generally less expensive upfront.
- Cons: More susceptible to damage from deep discharge, shorter lifespan compared to AGM, can leak if tipped, require more frequent maintenance for vented types.
Absorbed Glass Mat (AGM) Batteries
AGM batteries are a type of Valve-Regulated Lead-Acid (VRLA) battery. They are often called sealed, non-spillable, or dry cell batteries.
- Construction: The electrolyte is absorbed into fiberglass mats sandwiched between the lead plates, preventing spills and allowing for installation in various orientations. They are sealed and have pressure-activated valves to release gas only in overcharging situations.
- Maintenance: Maintenance-free due to their sealed design.
- Pros: Longer lifespan, better performance in extreme temperatures, more resistant to vibration and deep discharge, can be mounted in any orientation, safer due to spill-proof design, faster recharge rates.
- Cons: More expensive upfront cost.
Important Note: Wet cell and AGM batteries are not interchangeable. Your vehicle is designed to use a specific type of battery. Consult your owner’s manual to determine the correct battery type for your car.
Learn More