Chimeric Antigen Receptor (CAR) therapy has revolutionized cancer treatment, standing as the fourth pillar alongside surgery, radiation, and chemotherapy. This is particularly evident in metastatic cancers, once deemed incurable, where immunotherapy offers the prospect of long-term remission and potential cures. While CAR T cell therapy has achieved significant breakthroughs, especially in hematological malignancies, limitations persist, particularly in solid tumors. CAR Natural Killer (NK) cell therapy is now emerging as a compelling alternative, addressing many of these challenges and showing promise for a broader range of cancers. This article delves into the reasons why CAR NK cells are increasingly viewed as a potentially superior approach to CAR T cells in the evolving landscape of cancer immunotherapy.
CAR T Cell Therapy: Successes and Shortcomings
CAR T cell therapy involves genetically modifying a patient’s T cells to express CARs, synthetic receptors that redirect them to target and destroy cancer cells. Currently, five CAR T cell therapies have received FDA approval, all targeting B-cell antigens for hematological cancers. These therapies have shown remarkable success rates in relapsed or refractory B-cell lymphomas, leukemias, and multiple myeloma.
Table 1: Current FDA Approvals of CAR T Therapies
| CAR T Therapy | Target Antigen | Indication | Co-stimulatory Domain |
|---|---|---|---|
| Tisagenlecleucel | CD19 | B-cell precursor ALL (≤25 years, r/r), r/r large B-cell lymphoma | 4-1BB |
| Axicabtagene ciloleucel | CD19 | Large B-cell lymphoma (r/r), r/r follicular lymphoma | CD28 |
| Brexucabtagene autoleucel | CD19 | r/r mantle cell lymphoma | CD28 |
| Lisocabtagene maraleucel | CD19 | r/r large B-cell lymphoma | 4-1BB |
| Idecabtagene vicleucel | BCMA | r/r multiple myeloma | 4-1BB |
Despite these successes, CAR T cell therapy faces significant limitations, particularly when expanding beyond hematological malignancies to solid tumors. These challenges include:
- Toxicity: CAR T cell therapy is associated with severe toxicities like Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), limiting its broader application.
- Manufacturing Complexities: CAR T cell therapy typically relies on autologous T cells, requiring a patient-specific manufacturing process that is time-consuming, expensive, and can be challenging for patients with compromised immune systems.
- Solid Tumor Penetration: CAR T cells often struggle to effectively infiltrate solid tumors due to physical barriers like dense extracellular matrix and immunosuppressive tumor microenvironments (TME).
- Antigen Escape: Cancer cells can evade CAR T cell therapy by downregulating or losing the target antigen, leading to relapse.
- Limited Efficacy in Solid Tumors: While effective in hematological cancers, CAR T cell therapy has shown limited success in solid tumors, representing the vast majority of cancer types.
Fig. 1: Timeline of CAR T Therapy FDA Approvals
These limitations have spurred the exploration of alternative cell-based immunotherapies, and CAR NK cells are emerging as a particularly promising candidate.
CAR NK Cells: Overcoming CAR T Cell Limitations
CAR NK cells leverage the inherent advantages of Natural Killer cells, a distinct subset of cytotoxic lymphocytes in the innate immune system. NK cells offer several key benefits over T cells in the context of CAR therapy, directly addressing many of the shortcomings of CAR T cells.
1. Reduced Risk of Severe Toxicities:
One of the most compelling advantages of CAR NK cells is their significantly lower risk of inducing severe toxicities like CRS and ICANS. Clinical trials with CAR NK cells have shown a remarkably benign safety profile, with minimal to no CRS or neurotoxicity observed. This is attributed to the different cytokine profiles released by NK cells upon activation. Unlike CAR T cells, which release high levels of inflammatory cytokines such as TNF-α, IL-1β, IL-2, and IL-6, CAR NK cells primarily secrete cytokines like GM-CSF, with less pronounced release of pro-inflammatory cytokines associated with severe toxicities. This inherent safety advantage makes CAR NK cell therapy potentially applicable to a broader patient population, including those who might be ineligible for CAR T cell therapy due to comorbidities or risk factors for severe toxicities.
2. “Off-the-Shelf” Allogeneic Potential:
CAR NK cells offer the transformative potential of “off-the-shelf” allogeneic therapy. Unlike CAR T cells, which often require autologous cells due to the risk of Graft-versus-Host Disease (GVHD), CAR NK cells derived from allogeneic sources, such as umbilical cord blood, NK-92 cell lines, or induced pluripotent stem cells (iPSCs), show minimal risk of GVHD. This is because NK cell activation is not primarily MHC-dependent, and NK cells exhibit limited alloreactivity in the CAR setting. The ability to utilize allogeneic sources eliminates the need for patient-specific manufacturing, drastically reducing treatment costs, manufacturing time, and logistical complexities. “Off-the-shelf” CAR NK cell therapies can be readily available for immediate administration, overcoming manufacturing delays that can hinder CAR T cell therapy, especially in rapidly progressing cancers.
3. Enhanced Safety Mechanisms and Multiple Anti-Tumor Pathways:
Beyond reduced toxicities, NK cells possess intrinsic safety mechanisms and multiple pathways for tumor cell killing, further enhancing their appeal in CAR therapy. NK cells are naturally equipped with multiple receptors that regulate their activity, including inhibitory Killer Immunoglobulin-like Receptors (KIRs) that recognize MHC class I molecules on normal cells, preventing autoimmunity. Cancer cells often downregulate MHC class I, making them more susceptible to NK cell-mediated killing – a phenomenon known as “missing-self” recognition. Furthermore, NK cells mediate antibody-dependent cell-mediated cytotoxicity (ADCC) through CD16 receptors, enabling them to target antibody-coated cancer cells. These multiple anti-tumor pathways, combined with the CAR-mediated targeting, provide a multi-faceted approach to cancer eradication, potentially mitigating antigen escape and enhancing overall efficacy.
4. Improved Tumor Infiltration and Microenvironment Modulation:
While CAR T cells can face challenges infiltrating solid tumors, NK cells exhibit inherent migratory capacity and can potentially modulate the tumor microenvironment more effectively. NK cells are naturally recruited to tumors and can interact with various components of the TME. While research is ongoing to optimize CAR NK cell infiltration, the inherent properties of NK cells suggest they may be better suited to navigate the complex landscape of solid tumors compared to T cells. Furthermore, NK cells can release cytokines and chemokines that can reshape the TME, potentially making it more conducive to anti-tumor immunity.
5. CAR NK Cell Construct Flexibility and Optimization:
CAR NK cell constructs can be optimized to leverage NK cell-specific signaling pathways, potentially enhancing their cytotoxicity and persistence. While initial CAR NK cell designs often mirrored CAR T cell constructs using CD3ζ signaling domains, research is increasingly focusing on incorporating NK-specific intracellular signaling domains, such as DAP10, DAP12, and 2B4. These NK-specific domains can more effectively harness NK cell activation pathways, leading to improved target cell killing and cytokine production. Ongoing research is dedicated to identifying the optimal CAR constructs for NK cells to maximize their therapeutic potential.
Table 2: Comparison of CAR T, NK, and Macrophages
| Feature | CAR T Cells | CAR NK Cells | CAR Macrophages |
|---|---|---|---|
| Source | Autologous T cells | Allogeneic NK cells (Cord blood, NK-92, iPSC) | Autologous/Allogeneic Macrophages (iPSC-derived) |
| GVHD Risk | High | Minimal | Minimal |
| CRS/Neurotoxicity Risk | High | Low to None | Potential, but less defined |
| “Off-the-Shelf” Potential | Limited | High | Moderate (iPSC-derived potential) |
| Tumor Infiltration | Can be Challenging in Solid Tumors | Potentially Better | Naturally High in Many Solid Tumors |
| Manufacturing Complexity | Complex, Patient-Specific | Simpler, Scalable | Complex, but advancing |
| Persistence | Variable, Can be Long-Term | Shorter Lifespan, Repeat Dosing Possible | Potentially Long-Term, Tissue Resident Potential |
| Primary Mechanism | Cytotoxicity | Cytotoxicity, ADCC, “Missing-Self” Recognition | Phagocytosis, Antigen Presentation |
| Clinical Validation | Approved Therapies for Hematological Cancers | Early Phase Clinical Trials Promising | Preclinical, Phase I Trial Underway |
Fig. 2: CAR Structure for CAR T, CAR NK, and CAR Macrophage
Current Status of CAR NK Cell Therapy and Clinical Trials
CAR NK cell therapy is rapidly advancing in clinical development, with over 24 clinical trials planned or ongoing, primarily in Phase I/II stages. These trials are exploring CAR NK cells for a range of hematological malignancies and solid tumors. One notable Phase I clinical trial using cord blood-derived CAR NK cells targeting CD19-positive lymphoid tumors demonstrated remarkable efficacy and safety. In this trial, none of the 11 patients experienced CRS, neurotoxicity, HLH, or GVHD. Impressively, 73% of patients achieved an objective response, including a 64% complete remission rate. These early clinical results underscore the potential of CAR NK cells to deliver effective cancer therapy with a significantly improved safety profile compared to CAR T cells.
Table 3: Clinical Trials with CAR NK Cells (Examples)
| Target Antigen | Malignancy | Cell Source | Status |
|---|---|---|---|
| CD19 | B-cell Lymphoid Tumors | Cord Blood | Phase I |
| CD33/LL-1RAP | Acute Myeloid Leukemia (AML) | NK-92 Cell Line | Phase I/II |
| GD2 | Neuroblastoma, Melanoma | NK-92 Cell Line | Phase I |
| EGFR | Solid Tumors | NK-92 Cell Line | Phase I |
| HER2 | HER2+ Solid Tumors | NK-92 Cell Line | Phase I |
Table 4: Summary of a Phase I CAR NK Cell Clinical Trial Targeting CD19
| Feature | Result |
|---|---|
| Target Antigen | CD19 |
| Malignancy | B-lymphoid malignancies (CLL, Lymphoma) |
| Cell Source | Cord Blood-derived NK cells |
| GVHD | None |
| CRS | None |
| Neurotoxicity | None |
| Objective Response Rate | 73% (8/11 patients) |
| Complete Remission Rate | 64% (7/11 patients) |
| CAR NK Cell Persistence | Up to 12 months |
Fig. 3: Harnessing NK Cells for Cancer Immunotherapy
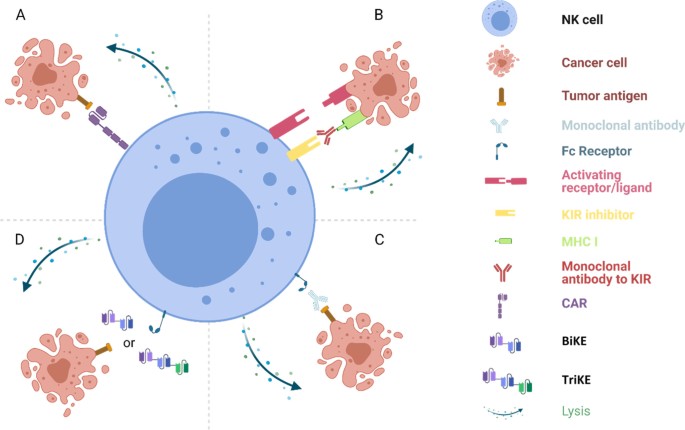 Fig. 3 Harnessing NK cells for cancer immunotherapy
Fig. 3 Harnessing NK cells for cancer immunotherapy
Addressing CAR NK Cell Limitations and Future Directions
While CAR NK cells offer significant advantages, challenges remain. Similar to CAR T cells, CAR NK cell therapy faces hurdles such as target antigen selection, antigen heterogeneity, tumor infiltration (though potentially less so), and immunosuppressive TMEs. Active research is focused on:
- Optimizing CAR NK Cell Manufacturing and Persistence: Strategies to enhance CAR NK cell expansion, persistence, and “off-the-shelf” production are crucial for wider accessibility and efficacy.
- Improving Tumor Infiltration and TME Modulation: Engineering CAR NK cells with chemokine receptors, resistance to immunosuppressive factors, or the ability to remodel the TME are being explored to enhance their activity in solid tumors.
- Combination Therapies: Combining CAR NK cell therapy with other immunotherapies, such as immune checkpoint inhibitors or oncolytic viruses, is a promising avenue to overcome resistance mechanisms and enhance anti-tumor responses.
- Next-Generation CAR NK Constructs: Developing novel CAR constructs that incorporate NK-specific signaling domains and functionalities to further enhance their potency and safety.
Conclusion: CAR NK Cells – A Paradigm Shift in Cancer Immunotherapy?
CAR NK cells are emerging as a compelling and potentially superior alternative to CAR T cells, particularly in the context of solid tumors and broader applicability. Their inherent safety profile, “off-the-shelf” allogeneic potential, multiple anti-tumor mechanisms, and adaptability position them as a transformative force in cancer immunotherapy. While CAR T cells have established the therapeutic potential of CAR therapy, CAR NK cells hold the promise of expanding this revolution, offering safer, more accessible, and potentially more effective treatments for a wider spectrum of cancers. As clinical trials progress and research deepens, CAR NK cells are poised to reshape the landscape of cancer immunotherapy, bringing us closer to more broadly applicable and curative cell-based cancer treatments.
Table 5: Current Status of CAR Macrophage Studies (Reference for Context)
| CAR Target | Target Antigen | Cell Source | Preclinical/Clinical |
|---|---|---|---|
| CAR-CD19/CD22 | CD19, CD22 | Macrophages | Preclinical |
| CAR-HER2 | HER2 | Macrophages | Preclinical, Phase I Trial (NCT04660929) |
| CAR-CCR7 | CCR7 | Macrophages | Preclinical |
| CAR-ECM (CD147-HER2) | HER2, ECM | Macrophages | Preclinical |
| CAR-iMACs (CD86-FcRγ) | Not Specified | iPSC-derived Macrophages | Preclinical |